Which Are The Most Critical Parameters To Control The Oxide Growth. Why?
Adjacent: 2.5 Nitrided Oxide Films Up: 2. Physics of Thermal Previous: 2.3 Rapid Thermal Oxidation
Subsections
- 2.iv.1 Oxidant Species
- ii.4.ii Influence of Temperature
- 2.four.three Influence of Pressure
- 2.4.iv Influence of Crystal Orientation
2.4 Oxidation Parameters
The desired characteristics and requirements of the fabricated oxide can be mainly influenced past the used oxidant species. For a chosen oxidant species the oxide growth rate usually is controlled past the temperature. Additionally, it is possible to vary the hydrostatic pressure in the reaction chamber, if the oxidation system offers such possibilities. Furthermore, the oxidation rate is also influenced by the crystal orientation of the used silicon substrate.
two.four.1 Oxidant Species
The most of import characteristic of oxidant molecules is that they comprise oxygen atoms, which are needed for the transformation from silicon to SiO![]() . The classical oxidant species are pure oxygen, which is too declared as dry out oxidation, and water vapour, which is also declared equally wet oxidation. In the middle of the 70's people started to mix pure oxygen generally with Chlorine or Hydrocloric Acid to improve oxide quality and speed upwardly growth charge per unit. The country of the art are nitrided oxides for MOS-gates, which are in principle as well produced past dry oxidation. Because of their extension and importance this species is described separately in Section 2.5
. The classical oxidant species are pure oxygen, which is too declared as dry out oxidation, and water vapour, which is also declared equally wet oxidation. In the middle of the 70's people started to mix pure oxygen generally with Chlorine or Hydrocloric Acid to improve oxide quality and speed upwardly growth charge per unit. The country of the art are nitrided oxides for MOS-gates, which are in principle as well produced past dry oxidation. Because of their extension and importance this species is described separately in Section 2.5
2.iv.1.1 Dry out Oxidation
During dry oxidation the silicon wafer is settled to a pure oxygen gas temper (O![]() ). The oxidation rate is low (< 100 nm/hr) and so the final oxide thickness can be controlled accurately. Compared with other oxides the dry oxide has the all-time cloth characteristics and quality. The chemical reaction between silicon (solid) and oxygen (gas) is
). The oxidation rate is low (< 100 nm/hr) and so the final oxide thickness can be controlled accurately. Compared with other oxides the dry oxide has the all-time cloth characteristics and quality. The chemical reaction between silicon (solid) and oxygen (gas) is
| | (2.i) |
With dry oxidation normally high quality thin oxide films up to 100nm thickness are produced. Dry oxides are especially used as gate oxides in MOS technology. The actually fabricated gate oxide thickness is in the magnitude of well-nigh only 2nm in the currently used 90nm process technology, whereas the exact thickness depends on the respective manufacturing setup. Unfortunately, at such thicknesses SiO
2.four.1.2 Wet Oxidation
During wet oxidation the silicon wafer is settled to a water vapour atmosphere (H![]() O). Moisture oxides grow really fast compared to dry out oxidation, which is the biggest advantage. The reason for the much higher growth rate is the oxidant solubility limit in SiO
O). Moisture oxides grow really fast compared to dry out oxidation, which is the biggest advantage. The reason for the much higher growth rate is the oxidant solubility limit in SiO![]() , which is much higher for moisture (H
, which is much higher for moisture (H![]() O) than for dry oxidation (O
O) than for dry oxidation (O![]() ). For 1000
). For 1000![]() C the typical solubility limit value is 5.ii
C the typical solubility limit value is 5.ii![]() ten
ten![]() cm
cm![]() for dry out oxidation compared to iii
for dry out oxidation compared to iii![]() ten
ten![]() cm
cm![]() for wet oxidation, which is well-nigh 600 times higher.
for wet oxidation, which is well-nigh 600 times higher.
Therefore, wet oxidation is applied for thick oxides in insulation and passivation layers, where thick oxide buffers are needed to suppress electric currents or to ensure loftier threshold voltage of parasitic transistors. The chemical reaction is
| | (2.two) |
Considering of its water content, moisture oxide films showroom a lower dielectric strength and more porosity to impurity penetration than dry out oxides. Therefore, wet oxidation is used when the electrical and chemic backdrop of the moving-picture show are not critical.
2.4.1.3 Mixed Flows of O 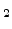 with H
with H 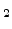 O, HCL, and Cl
O, HCL, and Cl 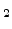
The gas flow of O![]() tin exist mixed in the furnace with H
tin exist mixed in the furnace with H![]() O, HCL, and Cl
O, HCL, and Cl![]() to go acceptable oxide quality at a higher growth rate. Besides a college growth rate, Hydrocloric Acid (HCL) or Chlorine (Cl
to go acceptable oxide quality at a higher growth rate. Besides a college growth rate, Hydrocloric Acid (HCL) or Chlorine (Cl![]() ) is often used in oxidation in club to prevent metallic contamination and to help avoiding defects in the oxidation layer [31]. HCL and Cl
) is often used in oxidation in club to prevent metallic contamination and to help avoiding defects in the oxidation layer [31]. HCL and Cl![]() take a cleaning effect of the furnace every bit well as an comeback of the oxide reliability. This ways that HCL and Cl
take a cleaning effect of the furnace every bit well as an comeback of the oxide reliability. This ways that HCL and Cl![]() additions provide benefits to the resulting device structures such as better ion passivation, higher and more compatible oxide dielectric strength, and improved junction properties due to lower current leakage.
additions provide benefits to the resulting device structures such as better ion passivation, higher and more compatible oxide dielectric strength, and improved junction properties due to lower current leakage.
The mixed flows were investigated among others by Bargain and Hess in the late seventy's, especially for the influence on the growth charge per unit. The addition of H![]() O every bit well every bit Cl is investigated in [32], and of HCL in [33]. In order to see the effect of the different mixed flows on the growth rate in a clear manner, the oxide thickness over time for a (100) oriented Silicon at yard
O every bit well every bit Cl is investigated in [32], and of HCL in [33]. In order to see the effect of the different mixed flows on the growth rate in a clear manner, the oxide thickness over time for a (100) oriented Silicon at yard![]() C is plotted in Figs. 2.7-2.9. It is notable that a double logarithmic scale of the plots leads to nearly linear curves also for the mixtures.
C is plotted in Figs. 2.7-2.9. It is notable that a double logarithmic scale of the plots leads to nearly linear curves also for the mixtures.
| |
| |
| |
The mixture of H
The chemic reaction of HCL with oxygen is
| | (two.3) |
Now it can be said that 2 moles of HCL produce 1 mol of H
In wet oxidation the addition of HCL does non increase the oxidation rate, rather the oxidation rate is decreased for the same percentage as the corporeality of HCL is added [34]. In H![]() O-HCL ambients the thickness uniformity and appearance of these oxides were considerably better than in pure H
O-HCL ambients the thickness uniformity and appearance of these oxides were considerably better than in pure H![]() O ambients. Likewise the defects in the oxide are considerably reduced.
O ambients. Likewise the defects in the oxide are considerably reduced.
ii.iv.2 Influence of Temperature
The oxidation rate increases significantly with the temperature in the furnace for dry besides every bit for wet oxidation. The temperature dependence of the oxidation rate is plotted in Fig. 2.xi for dry out and Fig. two.12 for wet oxidation. For wet oxidation in Fig. two.12 it can be seen that 100![]() C more than temperature leads to approximately double the oxidation rate, if the temperature is increased from 900 to thou
C more than temperature leads to approximately double the oxidation rate, if the temperature is increased from 900 to thou![]() C. The important temperature upshot can likewise exist observed for dry oxidation in Fig. 2.11, where the same temperature increase from 900 to 1000
C. The important temperature upshot can likewise exist observed for dry oxidation in Fig. 2.11, where the same temperature increase from 900 to 1000![]() C leads to much more double the oxidation rate.
C leads to much more double the oxidation rate.
| |
| |
The master reason of this striking temperature influence on the oxidation rate is the temperature dependence of the diffusivity of oxygen (O
2.4.3 Influence of Pressure
The oxidation rate increases with the hydrostatic pressure in the furnace for dry and wet oxidation in nearly the same way. The primary advantages of higher force per unit area oxidation over conventional atmospheric oxidation are the faster oxidation rate (see Fig. ii.xiii) and the lower processing temperature generally employed [35,36]. Both lead to less impurity improvidence and minimum junction move during the several oxidation steps which are necessary in the manufacturing of loftier-density multilayer IC devices. The quality and integrity of higher force per unit area oxides take been found to be comparable to atmospheric oxides. Oxidation-induced stacking faults are significantly reduced with higher pressure oxidation [37], which leads to improved device performance.
| |
2.4.4 Influence of Crystal Orientation
The studies of oxidation take shown that the oxidation rate also depends on the crystal orientation of the silicon substrate. Experiments have demonstrated many times that the oxide growth is faster on (111) oriented surfaces than on (100) oriented at any temperature for dry likewise as wet oxidation. Furthermore, equally plotted in Fig. ii.xiv for wet oxidation, information technology was found that the (111) and (100) orientation represent the upper and the lower jump for oxidation rates, respectively. Therefore, the growth rate for all other orientations lies between these 2 extremal values [38].
It is of import to understand orientation effects on oxidation more generally because many structures actually utilise etched trenches and other shaped silicon regions equally function of their structure. Ligenza [39] suggested that the crystal orientation effect might be caused by differences in the surface density of silicon atoms on the various crystal faces. He argued that since silicon atoms are required for the oxidation process, crystal planes that have higher densities of atoms should oxidize faster. Furthermore, he argued that not simply the number of silicon atoms per cm![]() is important, only besides the number of bonds matter, since information technology is necessary for Si-Si bonds to be broken for proceeding the oxidation. Ligenza calculated the ``available'' bonds per cm
is important, only besides the number of bonds matter, since information technology is necessary for Si-Si bonds to be broken for proceeding the oxidation. Ligenza calculated the ``available'' bonds per cm![]() on the various silicon surfaces and concluded that oxidation rates in H
on the various silicon surfaces and concluded that oxidation rates in H![]() O ambients should be in the order (111)>(100), which was also observed experimentally.
O ambients should be in the order (111)>(100), which was also observed experimentally.
| |
Side by side: 2.five Nitrided Oxide Films Up: 2. Physics of Thermal Previous: ii.3 Rapid Thermal Oxidation
Ch. Hollauer: Modeling of Thermal Oxidation and Stress Effects
Which Are The Most Critical Parameters To Control The Oxide Growth. Why?,
Source: https://www.iue.tuwien.ac.at/phd/hollauer/node14.html
Posted by: lamberttherad.blogspot.com

![\includegraphics[width=0.6\linewidth,bb=29 52 706 528, clip]{H2O/h2O}](https://www.iue.tuwien.ac.at/phd/hollauer/img52.png)
![\includegraphics[width=0.6\linewidth,bb=29 52 706 528, clip]{Cl2/clb}](https://www.iue.tuwien.ac.at/phd/hollauer/img53.png)
![\includegraphics[width=0.6\linewidth,bb=29 52 706 528, clip]{HCL/hcl}](https://www.iue.tuwien.ac.at/phd/hollauer/img54.png)
![\includegraphics[width=0.6\linewidth,bb=29 52 706 528, clip]{curv/deal/dryorient100}](https://www.iue.tuwien.ac.at/phd/hollauer/img57.png)
![\includegraphics[width=0.6\linewidth,bb=29 52 706 528, clip]{curv/deal/wet}](https://www.iue.tuwien.ac.at/phd/hollauer/img58.png)
![\includegraphics[width=0.6\linewidth,bb=29 52 706 528, clip]{curv/deal/pres}](https://www.iue.tuwien.ac.at/phd/hollauer/img61.png)
![\includegraphics[width=0.75\linewidth,bb=29 52 706 528, clip]{curv/deal/orient2}](https://www.iue.tuwien.ac.at/phd/hollauer/img62.png)

0 Response to "Which Are The Most Critical Parameters To Control The Oxide Growth. Why?"
Post a Comment